.png)
Unlock a Brighter Future: Comprehensive SLE Treatment Options Worldwide
Living with Systemic Lupus Erythematosus (SLE), commonly known as lupus, can be incredibly challenging. This complex autoimmune disease causes your body's immune system to mistakenly attack its own tissues and organs, leading to a wide range of symptoms that can affect your joints, skin, kidneys, brain, heart, and lungs. The unpredictable nature of lupus, with its cycles of flares and remission, often leaves individuals searching for effective strategies to manage their condition and improve their quality of life.
If you're grappling with symptoms like persistent fatigue, unexplained joint pain, a distinctive butterfly-shaped rash across your face, or other systemic issues, understanding "what is SLE lupus" and exploring "can lupus be treated" are crucial first steps. While there's currently no cure for lupus, advanced medical treatments have transformed the outlook for many, allowing them to lead fuller, more active lives. These treatments focus on reducing inflammation, suppressing the overactive immune system, and preventing organ damage.
For those exploring options beyond their local healthcare systems, medical tourism for SLE treatment offers a compelling alternative. Patients worldwide are increasingly looking for "lupus treatment abroad" or seeking "cost-effective lupus care" in countries renowned for their specialized expertise and cutting-edge facilities. This comprehensive guide will delve into everything you need to know about SLE, from its symptoms and causes to available treatments, global cost comparisons, and why considering international care might be the right choice for you.
What are the common symptoms of Systemic Lupus Erythematosus (SLE) and how is it diagnosed?
SLE is often called "the great imitator" because its symptoms can mimic many other conditions, making diagnosis challenging. While symptoms can appear gradually or suddenly, they often include:
- Fatigue: Profound and persistent tiredness, even after adequate rest.
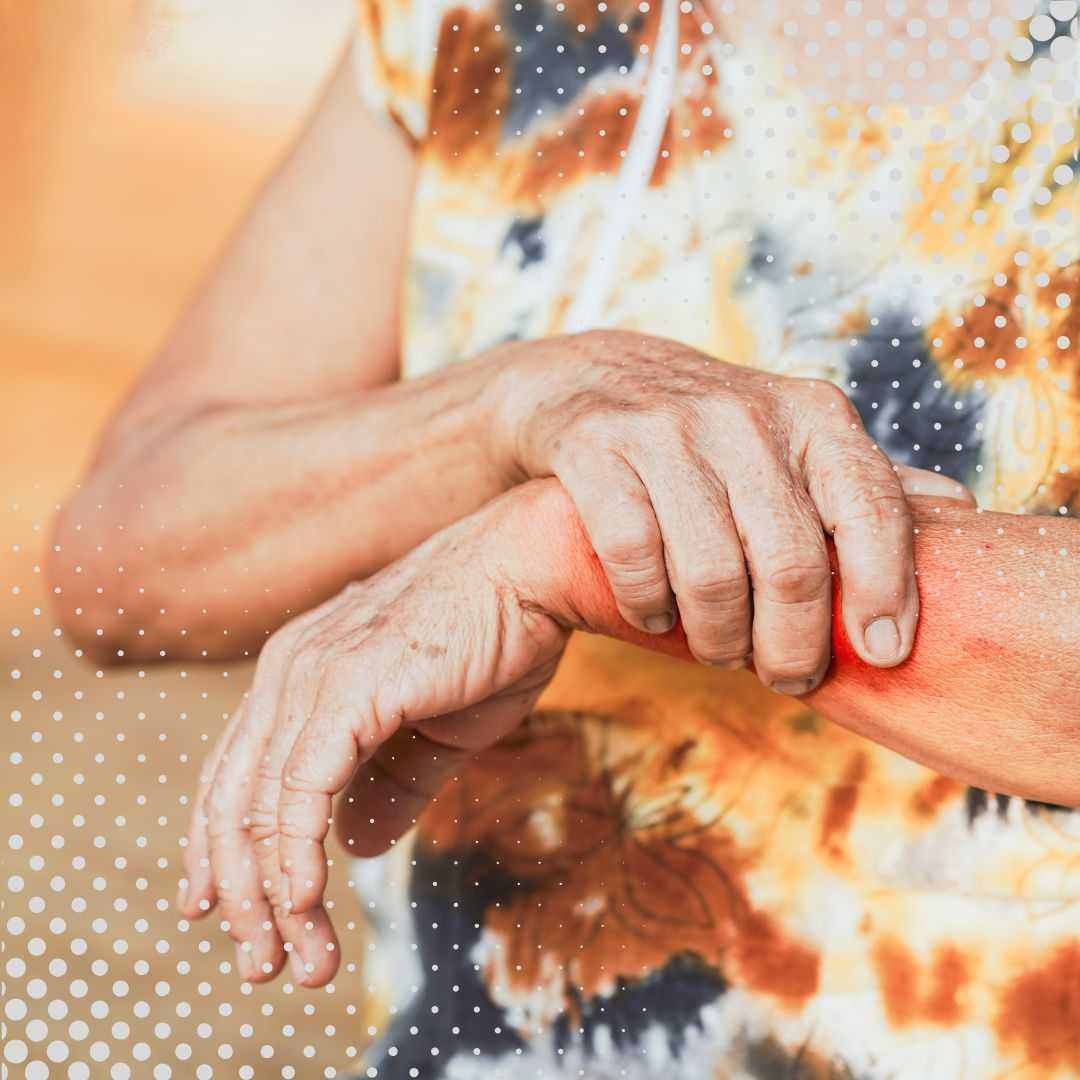
- Joint Pain and Swelling: Often in the hands, wrists, knees, and feet, usually affecting both sides of the body. You might search for "lupus joint pain relief."
- Skin Rashes: A characteristic "butterfly" (malar) rash across the bridge of the nose and cheeks, or disc-shaped rashes (discoid lupus). Sun sensitivity is also common, leading to worsening rashes. People often look for "lupus rash pictures" or "types of lupus rashes."
- Fever: Unexplained low-grade fever.
- Hair Loss: Often patchy or generalized.
- Raynaud's Phenomenon: Fingers and toes turning white or blue when exposed to cold or stress.
- Organ Involvement: Inflammation of the kidneys (lupus nephritis), heart (pericarditis), lungs (pleurisy), or brain (neuropsychiatric lupus).
Diagnosis typically involves a combination of your medical history, a physical exam, and various blood tests, including the Antinuclear Antibody (ANA) test, anti-dsDNA, and anti-Sm antibodies. Early diagnosis is key to effective management, which is why understanding "early signs of lupus" is so important.
What causes SLE and who is most at risk for developing it?
The precise "causes of lupus" remain largely a mystery, but scientists believe it results from a complex interplay of genetic predisposition and environmental triggers. It's not contagious and cannot be caught from another person.
Key risk factors include:
- Gender: Women are significantly more likely to develop lupus than men (about 9 times more often), particularly during their childbearing years (ages 15-44). Hormonal factors, especially estrogen, are thought to play a role.
- Genetics: A family history of lupus or other autoimmune diseases increases your risk, though most people with a genetic predisposition never develop the condition.
- Ethnicity: Lupus is more prevalent and often more severe in people of African American, Hispanic/Latino, Asian, and Native American descent.
- Environmental Triggers: These can "turn on" lupus in genetically susceptible individuals:
- Sunlight Exposure: Ultraviolet (UV) light can trigger skin lesions or internal lupus flares.
- Infections: Certain infections, like Epstein-Barr virus, have been linked to lupus.
- Medications: Some drugs, like certain blood pressure medications, anti-seizure drugs, and antibiotics, can induce a temporary form of lupus known as drug-induced lupus.
Understanding "lupus risk factors" helps individuals and doctors stay vigilant for symptoms.
What types of treatments are available for managing SLE symptoms and flares?
Managing SLE is a highly personalized journey, with treatment plans tailored to an individual's specific symptoms, severity, and organ involvement. The goal is to minimize symptoms, prevent flares, and reduce organ damage. Common categories of medication include:
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): For mild pain, fever, and inflammation (e.g., ibuprofen, naproxen).
- Antimalarial Drugs: Hydroxychloroquine (Plaquenil) is a cornerstone of lupus treatment, helping with fatigue, skin rashes, joint pain, and preventing flares. Many search for "hydroxychloroquine for lupus."
- Corticosteroids: Powerful anti-inflammatory and immunosuppressive drugs (e.g., prednisone) used for moderate to severe flares and organ involvement. They are highly effective but have significant side effects with long-term use.
- Immunosuppressants: Medications like methotrexate, azathioprine, and mycophenolate mofetil suppress the immune system to reduce inflammation and prevent organ damage, often used when corticosteroids are ineffective or to reduce their dosage.
- Biologics: Newer, targeted therapies that block specific immune pathways. Belimumab (Benlysta) was the first biologic specifically approved for SLE, and others like Anifrolumab (Saphnelo) are now available. Patients often look for "new lupus treatments" or "biologic drugs for lupus."
A rheumatologist specializes in managing SLE and will guide your treatment, often combining different therapies to achieve the best outcome.
Who is eligible for advanced SLE treatments, and what are their potential risks and benefits?
Eligibility for advanced SLE treatments, such as potent immunosuppressants or biologics, is typically determined by the severity of your condition, the extent of organ involvement, and whether you've responded adequately to conventional therapies (like antimalarials and low-dose steroids). Patients with active lupus nephritis (kidney inflammation), severe skin manifestations, or significant blood count abnormalities are often candidates.
Benefits: Advanced treatments can significantly reduce disease activity, prevent irreversible organ damage, minimize flare frequency and severity, and dramatically improve quality of life. For many, they can help achieve prolonged periods of remission, allowing them to resume normal activities.
Risks and Side Effects: While beneficial, these powerful medications come with potential risks:
- Increased Risk of Infection: By suppressing the immune system, you become more vulnerable to bacterial, viral, and fungal infections.
- Organ Toxicity: Some drugs can affect the liver or kidneys, requiring regular monitoring.
- Bone Density Loss: Long-term corticosteroid use can lead to osteoporosis.
- Cardiovascular Issues: SLE itself increases cardiovascular risk, and some treatments can exacerbate this.
- Digestive Issues: Nausea, vomiting, diarrhea are common with many immunosuppressants.
- Allergic Reactions: Biologic therapies carry a risk of infusion reactions or allergic responses.
Your doctor will carefully weigh these "risks of lupus treatment" against the potential benefits, emphasizing regular monitoring to mitigate adverse effects.
What is the expected recovery and long-term outlook for individuals managing SLE?
Unlike an acute illness with a clear recovery period, "living with lupus" involves ongoing management. There is no cure for SLE, but the goal of treatment is to achieve remission (a period with few to no symptoms) and prevent flares or organ damage. Modern medicine has dramatically improved the long-term outlook for people with SLE. Decades ago, a lupus diagnosis often meant a significantly shortened life expectancy; today, most people with SLE can expect to live a normal or near-normal lifespan.
Key aspects of the "lupus prognosis" and ongoing management include:
- Adherence to Treatment: Consistently taking prescribed medications, even during periods of remission.
- Regular Monitoring: Routine visits with your rheumatologist, blood tests, and imaging to track disease activity and monitor for side effects.
- Lifestyle Adjustments:
- Sun Protection: Avoiding sun exposure, using high-SPF sunscreen, and wearing protective clothing.
- Balanced Diet: Eating a healthy diet and potentially avoiding foods that trigger inflammation.
- Stress Management: Techniques like meditation, yoga, or therapy to cope with chronic illness.
- Regular Exercise: Gentle activity can improve joint mobility, reduce fatigue, and boost mood.
- Flare Management: Recognizing early signs of a flare-up and having a plan with your doctor to address it promptly.
While challenges remain, a proactive approach and a strong support system are vital for a good long-term outlook.
How much does SLE treatment cost globally, and can medical tourism offer affordable options?
The "lupus treatment cost" can be a significant burden, especially in countries with high healthcare expenses and limited insurance coverage. This is a primary driver for many patients to explore "medical tourism for lupus." Costs can vary dramatically based on the country, the specific medications (especially biologics), the duration of treatment, and whether hospitalization or specific procedures are required.
Here's a generalized cost comparison for managing SLE (monthly medication and specialist consultations, excluding severe flare-ups or hospitalization, which would be extra):
| Country | Estimated Monthly Cost (USD) | Notes |
|---|---|---|
| United States | $1,000 - $10,000+ | Highly variable based on insurance, medication (biologics are very expensive), and specialist fees. |
| Canada (Private Care) | $500 - $5,000+ | Public system covers much, but private costs for non-covered meds or faster access can be high. |
| United Kingdom (Private Care) | $400 - $4,000+ | NHS covers core, but private consultations and non-formulary biologics can be costly. |
| India | $150 - $1,500 | Significantly lower costs for consultations, generic medications, and even biologics. |
| Turkey | $200 - $2,000 | Modern facilities, skilled doctors, competitive pricing. |
| Mexico | $180 - $1,800 | Proximity for North Americans, good quality for various treatments. |
These figures are estimates and can fluctuate. The significant difference highlights why "cost of lupus treatment abroad" is a compelling search for many seeking quality care without financial strain.
Why should I consider traveling abroad for specialized SLE treatment and what are the advantages?
Considering "lupus treatment abroad" is a decision driven by several powerful advantages:
- Significant Cost Savings: As seen in the table above, the cost of advanced SLE treatments, including diagnostics, specialist consultations, and expensive biologic medications, can be drastically lower in medical tourism hubs compared to Western countries. This can translate to savings of 50-80% without compromising quality.
- Access to Advanced Therapies: Some countries are at the forefront of medical innovation, offering access to "new lupus treatments" or clinical trials that may not be readily available in your home country.
- Reduced Waiting Times: In many public healthcare systems, waiting lists for specialist appointments or advanced treatments can be long and frustrating. Traveling abroad often means quicker access to care, allowing you to start treatment sooner.
- Specialized Expertise: Renowned rheumatologists and specialized lupus centers in certain countries have extensive experience with complex cases, providing a fresh perspective or higher level of specialized care.
- Personalized Care and Attention: Many international clinics pride themselves on providing highly personalized patient experiences, with dedicated staff and comprehensive care plans.
- Privacy and Anonymity: For some, seeking medical care away from home offers a level of privacy they wouldn't have locally.
The "benefits of overseas treatment" for a chronic condition like SLE can be life-changing, offering hope and practical solutions.
Which countries are recognized for offering the best value and quality in SLE care for international patients?
When searching for "best countries for lupus treatment," several destinations consistently stand out for their blend of high-quality care, advanced facilities, experienced medical professionals, and competitive pricing:
- India: A global leader in medical tourism, India boasts numerous JCI-accredited hospitals with state-of-the-art rheumatology departments. Highly skilled doctors, advanced diagnostics, and significantly lower costs for medications and procedures make it a top choice.
- Turkey: With its strategic location, modern infrastructure, and growing number of internationally accredited hospitals, Turkey offers excellent value. It's particularly strong in areas of autoimmune disease management, attracting patients from Europe and the Middle East.
- Thailand: Known for its exceptional hospitality and world-class medical facilities, Thailand provides comprehensive care with a focus on patient experience. Bangkok, in particular, has several renowned hospitals with strong rheumatology programs.
- Mexico: Especially popular with patients from the United States and Canada due to its proximity, Mexico offers high-quality care at a fraction of the cost. Cities like Tijuana, Cancun, and Monterrey have established medical tourism sectors.
- South Korea: A rising star in medical tourism, South Korea offers incredibly advanced medical technology, highly trained specialists, and a strong emphasis on research and development, including new therapies for autoimmune conditions.
These countries often have a higher volume of patients seeking specific treatments, which means their specialists gain extensive experience, much like a specific type of car mechanic becomes an expert by working on that particular model daily.
What crucial steps should I take to plan and ensure a safe, high-quality SLE treatment journey abroad?
"How to plan a medical trip" for SLE requires careful consideration to ensure safety and quality:
- Thorough Research: Look for hospitals and clinics that specialize in rheumatology and have international accreditations (e.g., Joint Commission International - JCI). Read patient reviews and testimonials.
- Verify Doctor Credentials: Confirm the qualifications, experience, and board certifications of the rheumatologists who will be managing your care. Ensure they have experience with complex SLE cases.
- Gather Medical Records: Compile all relevant medical history, diagnostic test results, current medication lists, and a summary from your local doctor. Have them translated if necessary.
- Consult with Your Local Doctor: Discuss your plans to seek treatment abroad. They can offer advice, provide necessary referrals, and plan for your follow-up care upon return.
- Engage a Medical Tourism Facilitator: Companies like PlacidWay specialize in connecting patients with reputable international providers. They can assist with:
- Clinic and doctor selection.
- Travel logistics (flights, accommodation, visa assistance).
- Medical record translation and communication with the medical team.
- Cost estimates and payment coordination.
- Post-treatment follow-up arrangements.
- Understand the Treatment Plan and Costs: Get a clear, detailed treatment plan and a transparent breakdown of all costs upfront, including consultations, diagnostics, medications, and potential follow-up appointments.
- Travel Insurance: Purchase comprehensive travel and medical insurance that covers international medical treatment and any potential complications.
- Language and Cultural Considerations: Ensure there will be interpreters if needed and be prepared for cultural differences.
Taking these steps helps minimize risks and maximize the chances of a successful and positive experience when "traveling for medical treatment lupus."
What are the typical experiences and success stories of patients seeking SLE treatment internationally?
While individual outcomes vary, the collective experience of patients seeking SLE treatment abroad often reflects a journey of renewed hope and improved well-being. Many individuals, after struggling with limited options or prohibitive costs in their home countries, find substantial relief and a better quality of life through international medical care.
Common themes in "patient success stories" and experiences include:
- Significant Symptom Reduction: Patients frequently report a decrease in chronic pain, fatigue, and the frequency of lupus flares, allowing them to participate more fully in daily activities.
- Improved Organ Function: For those with organ involvement, such as lupus nephritis, successful treatment abroad often leads to stabilization or improvement in kidney function, preventing further damage.
- Access to Life-Changing Medications: Many patients finally gain access to advanced biologic therapies or specialized treatment protocols that were previously out of reach due to cost or availability.
- Empowerment and Education: International clinics often prioritize patient education, empowering individuals to better understand and manage their condition, leading to greater self-efficacy.
- Personalized and Holistic Care: Patients appreciate the attentive, comprehensive, and often more holistic approach to care, where their individual needs are prioritized, and treatment plans are tailored rather than generalized.
- Cost-Effectiveness and Financial Relief: The substantial savings realized often reduce a major source of stress, allowing patients to focus on their health rather than overwhelming medical bills.
- Positive Travel Experience: Beyond the medical aspect, many patients enjoy the cultural experience, often finding it therapeutic and enriching, contributing to their overall sense of well-being.
These experiences highlight that for many, seeking SLE treatment abroad is not just about a medical procedure, but about embarking on a transformative journey towards a healthier, more manageable life with lupus.
Take the Next Step with PlacidWay
Ready to explore treatment options abroad? Discover top clinics, compare prices, and get a free quote tailored to your needs with PlacidWay.
CAR-T Cell Therapy | Chimeric Antigen Receptor T-Cell








Share this listing