Contents
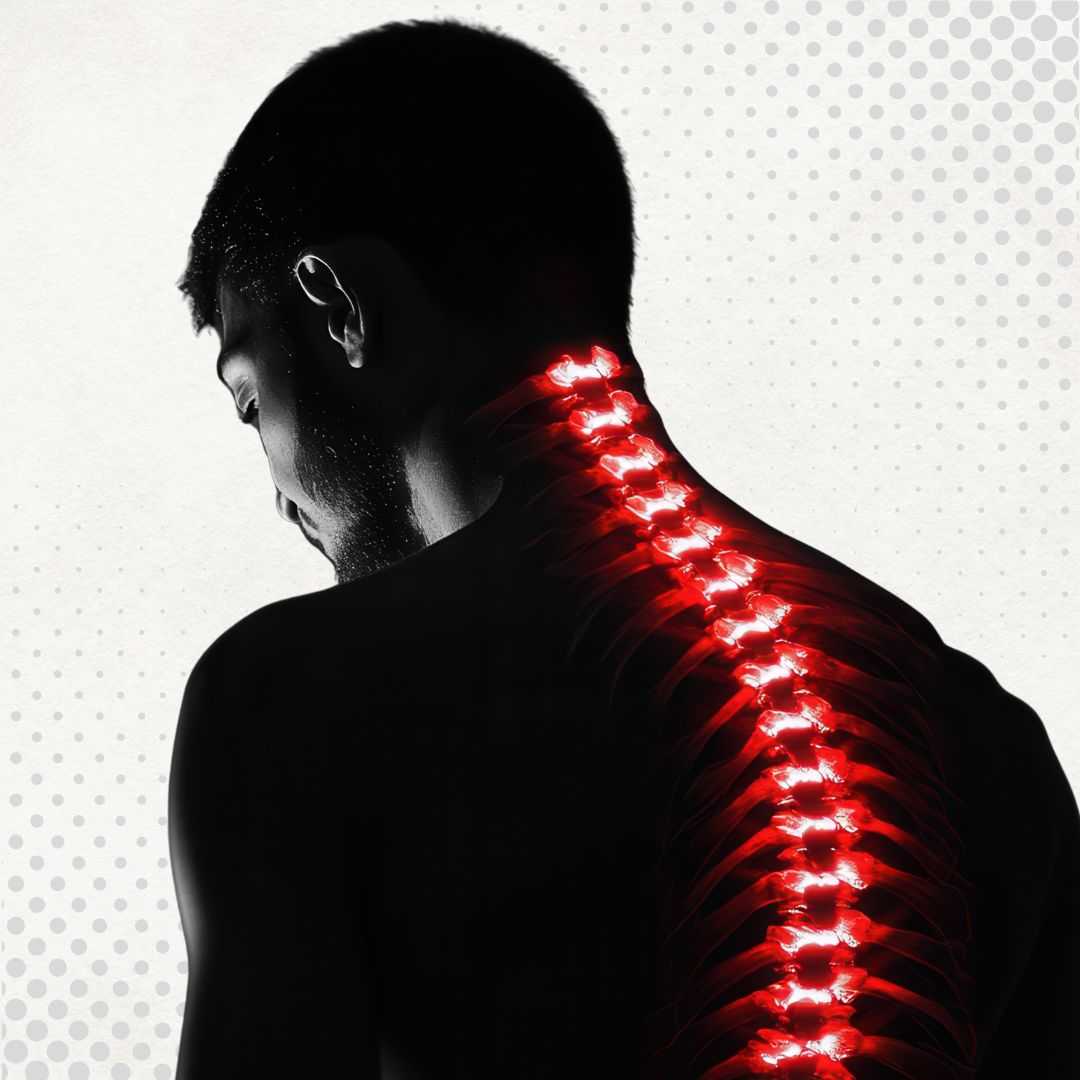
Regain Function with Japan Stem Cell Therapy for Spinal Cord Injury
Japan stands at the forefront of the global regenerative medicine revolution, particularly for complex conditions like Spinal Cord Injury (SCI). As the nation where induced pluripotent stem cells (iPSCs) were discovered, Japan has established one of the world's most advanced and strictly regulated environments for cell therapies. For patients seeking treatment for SCI, this means access to cutting-edge protocols that prioritize safety and scientific rigor above all else.
Therapy in Japan typically involves the use of Mesenchymal Stem Cells (MSCs), often derived from the patient's own bone marrow or adipose tissue, and increasingly, innovative therapies approved under Japan's unique "Act on the Safety of Regenerative Medicine." Unlike unregulated markets, clinics here must undergo rigorous government vetting to offer these procedures.
What makes the Japanese approach unique is the integration of advanced robotics during the rehabilitation phase. Treatment is rarely just about the injection; it is often combined with intensive physical therapy using Cyberdyne's HAL (Hybrid Assistive Limb) robotic suits, designed to amplify weak nerve signals and retrain the brain-body connection.
Choosing Japan for SCI treatment is a decision to pursue recovery in a healthcare system that blends futuristic innovation with meticulous, patient-centered care.
Did You Know?
Japan was the first country in the world to grant conditional approval for a stem cell product specifically for treating spinal cord injuries (Stemirac). This landmark decision allows patients to access therapies that have shown safety and probable efficacy much faster than in other regulatory environments.
Key Insights at a Glance
Therapies are regulated by the Ministry of Health, Labour and Welfare, ensuring high safety standards.
Treatment often integrates HAL (Hybrid Assistive Limb) exoskeletons to maximize neuroplasticity.
Japanese labs use proprietary 3D culture methods to increase the potency and survival rate of stem cells.
Japanese nursing care (Omotenashi) ensures meticulous attention to hygiene and patient comfort.
Home to Nobel Prize-winning research, driving continuous improvement in regenerative protocols.
Goals are realistic and functional—improving sensation, bladder control, or motor function, rather than promising impossible cures.
The "Sakigake" Designation
Japan has a special regulatory pathway called "Sakigake" (meaning "pioneer" or "forerunner"). This system fast-tracks the review process for innovative medical products that are developed in Japan, ensuring that ground-breaking stem cell therapies reach patients faster than anywhere else in the world.
Japanese medical packages for international patients are often premium and comprehensive. In this section, we showcase providers offering packages that typically include the stem cell cultivation and administration, extensive pre-treatment testing, and a dedicated rehabilitation program. Given the language barrier, top packages also invariably include medical translation services and a personal coordinator to guide you through the hospital experience.
Note: Packages in Japan focus heavily on quality assurance and safety testing of the cells before administration.
Regenerative medicine in Japan is an investment in cutting-edge science. The table below outlines estimated costs for various stem cell protocols (e.g., autologous MSCs from bone marrow or fat). While costs are higher than in Southeast Asia or Latin America, they reflect the stringent regulatory compliance, the cost of advanced cell culturing in GMP-grade facilities, and the high standard of medical care.
Tip: Treatment costs usually encompass multiple injections and a hospitalization period for monitoring.
Stem Cell Treatment for Spinal Cord Injury Cost Comparison in Japan
| Country | Procedure | Price |
|---|---|---|
| United States | Stem Cell Treatment for Spinal Cord Injury, Stem Cell Therapy | $50000 |
We have selected a list of clinics and hospitals in Tokyo, Osaka, and Kyoto that are officially licensed under the Regenerative Medicine Safety Act. These facilities are not just clinics but often research-driven institutions equipped with advanced cell processing centers (CPCs). Explore their profiles to verify their license numbers and see details about their specific cell culture technologies and rehabilitation facilities.
Safety First: Always verify that the clinic has a specific license for "Class I" or "Class II" regenerative medicine plans.
Navigating a medical journey in a foreign language can be daunting. The video testimonials below feature international patients who have traveled to Japan for SCI treatment. Listen to their experiences regarding the effectiveness of the therapy, the quality of the rehabilitation, and how they managed the cultural and linguistic aspects of their stay. Their stories provide a realistic picture of the recovery journey.
Insight: Patients often praise the cleanliness, organization, and politeness of the Japanese medical staff.
Patient reviews offer crucial transparency. In this section, we compile verified feedback from patients who have undergone regenerative procedures in Japan. Read about their improvements in motor function or sensation, their satisfaction with the clinic's administrative support, and their overall impression of the "Japanese standard of care." These reviews help set realistic expectations for your own treatment.
Review Tip: Look for comments detailing the rehabilitation phase, as this is critical for SCI recovery.
In Japan, stem cell therapy is the domain of highly specialized neurosurgeons and regenerative medicine experts. The specialists below are leaders in their field, often holding positions at prestigious universities and contributing to global research. Their profiles reflect a deep commitment to scientific integrity and patient safety.
Regenerative Neurosurgeons
Experts in CNS Repair
These specialists are board-certified neurosurgeons who have pivoted to regenerative medicine. They possess the intricate anatomical knowledge required for safe intrathecal injections and understand the complex pathology of spinal cord injuries.
Cell Culture Specialists (PhDs)
Laboratory Quality Control
Behind every successful treatment is a team of PhD scientists managing the Cell Processing Center (CPC). They ensure the stem cells are viable, sterile, and potent. In Japan, these experts strictly adhere to GCTP standards to guarantee the quality of the product.
Rehabilitation Physicians
Robotics & Neuro-rehab
These doctors specialize in physical medicine and rehabilitation (PM&R). They design personalized recovery programs, often incorporating advanced robotics like the HAL suit to help patients regain strength and coordination post-transplant.
Global Leadership in Science
Japan's reputation is built on solid scientific ground, underscored by Dr. Shinya Yamanaka's Nobel Prize for stem cell research. This culture of innovation permeates the clinical landscape, where treatments are based on evidence and rigorous data rather than marketing hype.
Patients come to Japan because they trust the science and the integrity of the medical system.
Unmatched Safety Standards
The "Act on the Safety of Regenerative Medicine" provides a level of patient protection found almost nowhere else. Clinics are strictly monitored, and cell processing facilities must meet pharmaceutical-grade standards.
This eliminates the fear of "rogue clinics" and ensures that the cells you receive are pure, sterile, and safe.
Technological Integration
Japan excels at combining biological therapies with technological support. The widespread availability of robotic rehabilitation tools (like Cyberdyne's HAL) allows for a synergistic approach to recovery.
This comprehensive care model—treating the biology with cells and the function with robotics—offers a more holistic path to improvement for SCI patients.
Accessing advanced medical care in Japan requires careful coordination and expert guidance. PlacidWay connects you with Japan's accredited regenerative medicine clinics, ensuring a seamless, safe, and supported experience.
Accreditation Checks
We verify that every partner clinic holds a valid license from the Ministry of Health, Labour and Welfare for regenerative medicine.
Language Support
We ensure all arranged appointments include professional medical interpretation, bridging the gap between you and your Japanese medical team.
Medical Coordination
We facilitate the transfer of your MRI scans and medical records to Japan for preliminary review by specialists before you travel.
Transparent Costs
We provide clear, itemized quotes that explain high-tech costs, ensuring you understand the investment without hidden fees.
Visa Assistance
Our team can assist in providing the necessary documents for a Medical Visa (Visa for Medical Stay), which is often required for extended treatments.
Explore the potential of regenerative medicine with confidence. Contact PlacidWay today to receive a personalized assessment and quote for stem cell therapy in Japan.
Get Your Free Personalized Quote













.png)

.png)
.png)





Share this listing