Key Takeaways
-
Advanced Regulation: Japan is one of the only countries with a national legal framework (ASRM) specifically guaranteeing the safety of regenerative medicine for both locals and foreigners.
-
Cultured Cells: Unlike the US, Japanese clinics are legally permitted to culture (expand) stem cells, allowing for doses of 100 million+ cells, which is critical for effective meniscus repair.
-
Cost Comparison:
-
Japan: $6,500 – $15,000 (Average for knee/meniscus packages)
-
USA: $8,000 – $25,000 (Often for lower-dose, uncultured cells)
-
Mexico: $3,500 – $8,000
-
Korea: $5,000 – $10,000
-
-
Success Metrics: Clinical data suggests 60–80% of patients experience significant pain reduction and improved function, with some studies showing meniscal tissue regeneration.
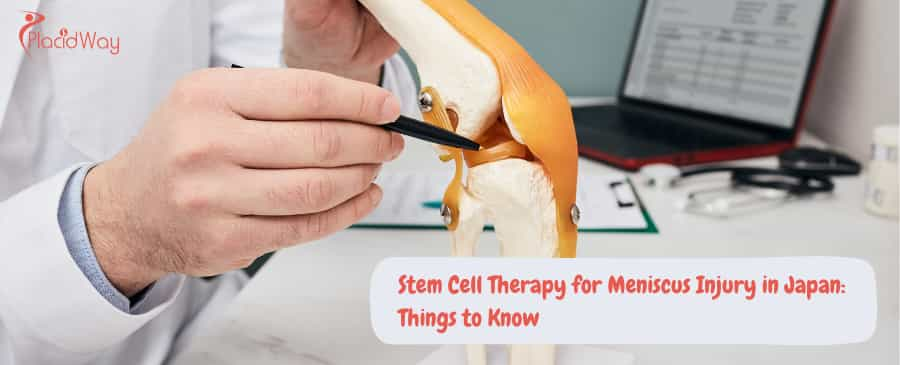
Why Choose Japan for Meniscus Regeneration?
Japan offers a unique combination of government-sanctioned safety protocols and advanced cellular technology that allows for high-dose cultured stem cell treatments not available in many Western nations.
While countries like Mexico and Turkey are popular for affordability, Japan stands alone as the global leader in high-tech, regulated regenerative medicine. The "Act on the Safety of Regenerative Medicine" (ASRM) ensures that every clinic offering stem cell therapy for meniscus tears is vetted by the Ministry of Health, Labour and Welfare (MHLW).
-
Technological Superiority: Japanese labs use proprietary culturing methods to multiply stem cells to over 100 million viable cells per dose.
-
Safety First: Treatments are classified by risk, and strict oversight prevents the "wild west" scenarios seen in less regulated markets.
-
Medical Tourism Infrastructure: Top clinics in Tokyo and Osaka provide full English support, medical visas, and concierge services.
Did You Know? Japan was the first country to fast-track regenerative medicine approvals, meaning therapies that might take 10 years to reach the US market are often already available and regulated in Japan.
The Procedure: How Meniscus Stem Cell Therapy Works
The process typically involves harvesting autologous (your own) fat tissue, culturing the cells in a lab for 3–5 weeks to boost their numbers, and then injecting them precisely into the knee joint.
Treating a meniscus injury (torn meniscus) in Japan is rarely a "one-day" procedure like in other countries. The focus is on cell potency.
-
Consultation & Screening: MRI review to confirm the tear type (degenerative vs. traumatic) and severity.
-
Harvesting (Visit 1): A mini-liposuction (usually abdominal fat) is performed under local anesthesia to collect adipose tissue. This takes about 30 minutes.
-
Culturing (The "Wait" Period): Your cells are sent to a Cell Processing Center (CPC). Over 3–5 weeks, they are expanded from a few thousand to 100–200 million mesenchymal stem cells (MSCs).
-
Injection (Visit 2): You return for the injection. The high-dose cells are injected into the knee joint, often guided by ultrasound. Some advanced clinics may use arthroscopy for precise placement.
Cost of Meniscus Stem Cell Therapy in Japan
Expect to pay between $6,500 and $15,000 USD for a complete cultured stem cell package for one knee, which includes processing, anesthesia, and doctor fees.
Pricing in Japan is higher than in Mexico but offers higher value due to the cell count and safety checks.
Global Cost Comparison: Knee & Meniscus Stem Cell Therapy
|
Country |
Average Price Range (USD) |
Cell Type Allowed |
Regulatory Status |
|---|---|---|---|
|
Japan |
$6,500 – $15,000 |
Cultured (High Dose) |
Strictly Regulated (MHLW) |
|
United States |
$5,000 – $25,000 |
Non-Cultured (Low Dose) |
FDA Restrictions |
|
South Korea |
$5,000 – $10,000 |
Cultured |
Regulated (Growing) |
|
Mexico |
$3,500 – $8,000 |
Cultured |
Mixed Regulation |
|
Turkey |
$3,000 – $6,000 |
Various |
Moderate Regulation |
Expert Insight: Be wary of clinics quoting over $30,000 for a simple knee procedure. Prices above $20,000 in Japan usually involve systemic IV treatments for anti-aging or neurological conditions, not localized orthopedic injections.
Success Rates and Realistic Expectations
Clinical data indicates a 60–80% success rate in pain reduction and functional improvement for meniscus injuries, though complete anatomical regeneration of a severed meniscus is rare.
Patients should view stem cell therapy as a way to manage pain, reduce inflammation, and heal micro-tears, rather than a magic eraser for a complex bucket-handle tear.
-
Best Candidates: Patients with degenerative meniscus tears, partial tears, or those looking to avoid a meniscectomy (removal of meniscus).
-
Pain Relief: Most patients report significant pain relief within 3 months post-injection.
-
Cartilage Protection: Studies suggest MSCs help preserve remaining cartilage, slowing the progression to osteoarthritis.
Important Note: If your meniscus is fully detached or blocking joint movement (locking), surgery might still be required before stem cell therapy.
Recovery Timeline and Aftercare
Recovery is minimal compared to surgery; most patients walk out the same day, but full tissue healing requires 3 to 6 months of gradual progression.
-
Day 1 (Injection): Mild swelling and stiffness are normal. You can walk, but rest is recommended.
-
Week 1: Avoid strenuous exercise. Light walking is encouraged to circulate joint fluid.
-
Month 1: Inflammation subsides. Stem cells are actively signaling repair. Begin low-impact physical therapy (cycling, swimming).
-
Month 3-6: Significant improvements in pain and range of motion. Return to impact sports should be guided by a follow-up MRI.
Risks and Safety Profile
The use of autologous (your own) cells minimizes rejection risk to near zero, with the primary risks being minor infection at the injection site or temporary swelling.
Because Japan strictly regulates Cell Processing Centers (CPCs), the risk of contamination—a serious concern in unregulated markets—is extremely low.
-
Infection? less than 0.1% risk when performed in sterile Japanese clinics.
-
Tumorigenesis: No validated cases of tumor formation from autologous adipose-derived MSCs used in orthopedic settings in Japan.
-
Swelling: Temporary joint swelling ("flare-up") occurs in about 10% of patients as a natural healing response.
FAQ: Common Questions About Meniscus Stem Cell Therapy in Japan
Is stem cell therapy legal for foreigners in Japan?
Yes, absolutely. The Act on the Safety of Regenerative Medicine (ASRM) allows licensed clinics to treat international patients. You can legally travel to Japan specifically for this treatment, and specialized "Medical Visas" are available for longer stays.
Can stem cells fully regrow a torn meniscus?
While stem cells can regenerate tissue and heal tears (especially in the "red zone" where blood supply exists), they cannot regrow a completely removed meniscus from scratch. They are best for repairing tears and improving the quality of the remaining tissue.
Does Japanese national insurance cover this for tourists?
No. Stem cell therapy for meniscus injury is considered an advanced/experimental medical treatment and is self-pay for international patients. It is not covered by Japanese National Health Insurance or standard travel insurance.
How many trips to Japan do I need?
Typically, two trips are required. Trip 1 is for fat harvesting (1-2 days). Trip 2 is for the injection (1-2 days), usually scheduled 4 weeks later. Some clinics allow you to stay in Japan for the month, while others coordinate the logistics for two separate visits.
Why is culturing stem cells important?
"Culturing" allows doctors to multiply your cells into the millions. A standard US treatment might provide 50,000 cells, whereas a cultured Japanese treatment provides 100,000,000+ cells. Higher cell counts are statistically linked to better outcomes in cartilage and meniscus repair.
Is it painful?
The procedure is minimally invasive. The fat harvesting is done under local anesthesia (you are awake but numb), and the knee injection feels similar to a standard cortisone shot. Most patients manage with over-the-counter pain relievers afterwards.
Ready to Explore Your Options?
Don't let knee pain dictate your life. Japan's world-class regenerative medicine offers a safe, regulated, and potent alternative to surgery.
PlacidWay connects you directly with Japan's top MHLW-approved stem cell clinics. We help you navigate:
-
Free Quote Comparisons: Get transparent pricing from multiple clinics.
-
Medical Visa Assistance: We guide you through the paperwork.
-
Tele-Consultations: Speak with a Japanese specialist before you fly.




.png)
.png)











Share this listing