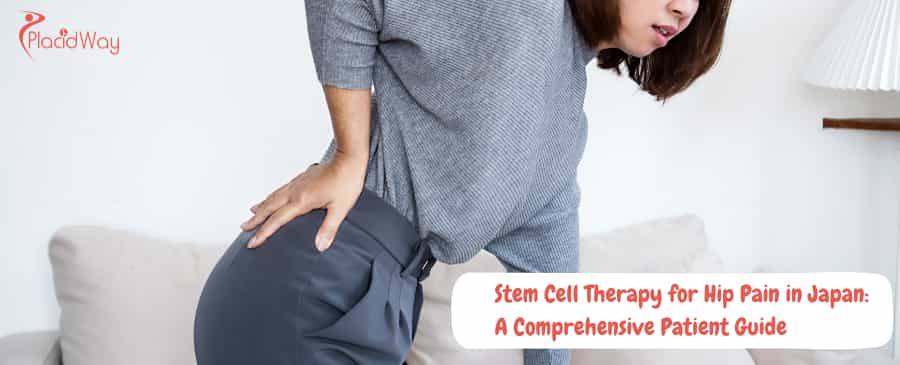
Hip pain from osteoarthritis (OA) or avascular necrosis (AVN) can be debilitating, often leaving patients with a difficult choice: live with chronic pain or undergo invasive total hip replacement surgery. Stem cell therapy for hip pain has emerged as a revolutionary non-surgical alternative, capable of regenerating damaged cartilage and reducing inflammation. Japan, with its Nobel Prize-winning history in regenerative medicine and strict government regulations, has become the global gold standard for these treatments.
This guide covers everything you need to know about receiving stem cell treatment for hips in Japan, comparing it with other popular destinations like Turkey and Mexico to help you make an informed medical tourism decision.
Key Takeaways
-
Japan’s Safety Advantage: Japan operates under the Act on the Safety of Regenerative Medicine (ASRM), ensuring all clinics are government-licensed and therapies are rigorously tested for safety.
-
High Potency: Japanese clinics often use cultured stem cells, allowing for hundreds of millions of cells to be injected, unlike the uncultured "same-day" systems common in the US.
-
Global Cost Comparison for Hip Stem Cell Packages:
-
Japan: $10,000 – $25,000 (Includes high-dose cultured cells, strict safety testing).
-
Mexico: $3,500 – $8,000 (Popular for US patients, variable regulations).
-
Turkey: $3,000 – $6,000 (Highly competitive pricing, often combines with rehab).
-
USA: $5,000 – $12,000 (Uncultured cells only, lower efficacy potential).
-
What is Stem Cell Therapy for Hip Pain?
Stem cell therapy is a regenerative procedure that uses your body's own potent cells to repair damaged hip cartilage, reduce inflammation, and improve joint function without surgery.
Unlike steroid injections that temporarily mask pain, stem cell therapy targets the root cause of hip dysfunction. By injecting concentrated Mesenchymal Stem Cells (MSCs) into the hip joint, the treatment stimulates the repair of worn cartilage and modulates the immune system to stop further degeneration.
Conditions Treated
-
Osteoarthritis (OA): Wear and tear of the hip joint cartilage.
-
Avascular Necrosis (AVN): Bone death due to lack of blood supply (Stages 1 & 2 respond best).
-
Labral Tears: Damage to the ring of cartilage surrounding the hip socket.
-
Bursitis: Chronic inflammation of the fluid-filled sacs in the hip.
Types of Stem Cells Used
-
Adipose-Derived (Fat): Harvested via mini-liposuction. These are preferred in Japan because they yield a massive number of MSCs when cultured.
-
Bone Marrow-Derived: Harvested from the pelvic bone. Often used for bone-related issues like AVN.
-
Umbilical Cord (Allogeneic): Donor cells used in some international clinics (e.g., Mexico), though Japan focuses heavily on autologous (your own) cells for safety.
Why Choose Japan? The "Things to Know" About Safety
Japan offers the world's most strictly regulated environment for regenerative medicine, guaranteeing that patients receive safe, pure, and effective cell products free from contamination.
While countries like Mexico and Turkey offer affordability, Japan offers certainty. In 2014, Japan enacted the Act on the Safety of Regenerative Medicine, the world's first law specifically designed to regulate stem cell therapies.
The "Japan Quality" Difference
-
Cultured Cells: Japanese law allows clinics to culture (grow) your stem cells in a lab for 3-4 weeks. This turns a sample of 1 million cells into 100 million+ cells, drastically increasing the therapy's potency.
-
Government Licensing: Every clinic must submit their treatment protocols to a government-certified committee for approval.
-
Traceability: Every cell sample is tracked from harvest to injection.
Did You Know? Japan is the birthplace of iPSC (Induced Pluripotent Stem Cell) technology, discovered by Nobel Laureate Shinya Yamanaka. This culture of innovation permeates their clinical treatments.
Candidates & Success Rates
Ideal candidates are those with mild to moderate osteoarthritis or early-stage AVN who wish to delay or avoid hip replacement surgery; success rates for pain relief often exceed 80%.
Not everyone is a candidate. If your hip is "bone-on-bone" (Stage 4 OA) or if the femoral head has collapsed (Stage 3-4 AVN), stem cells may not be able to regenerate the joint structure fully.
Who Qualifies?
-
Age: Generally 18–80 (older patients may require longer cell culture times).
-
Condition Severity: Mild to Moderate (Kellgren-Lawrence Grade 1-3).
-
Health Status: Free from active cancer or severe infections.
Expected Outcomes
-
Pain Reduction: 75%–85% of patients report significant pain relief within 3 months.
-
Cartilage Growth: MRI studies have shown thickening of cartilage in some patients, though functional improvement (walking, climbing stairs) is the primary goal.
-
Duration: Results typically last 3 to 5 years, after which a "booster" injection may be needed.
The Procedure: Step-by-Step
The Japanese protocol typically involves two visits: one for harvesting fat tissue and a second visit 3-4 weeks later for the injection of the high-dose cultured cells.
Step 1: Initial Consultation & Harvest (Visit 1)
You arrive in Japan for a 1-2 day trip. Doctors perform a thorough screening and a "mini-lipo" procedure (usually from the belly) to collect about 20cc of fat. This takes 30 minutes under local anesthesia.
Step 2: Cell Processing (The Waiting Period)
You return home. Over the next 3-4 weeks, the clinic's Cell Processing Center (CPC) isolates the stem cells and cultures them. They test for bacteria, viruses, and cancerous properties to ensure absolute safety.
Step 3: The Injection (Visit 2)
You return to Japan. The doctor injects the 100 million+ stem cells directly into the hip joint using ultrasound or fluoroscopic guidance to ensure pinpoint accuracy. You walk out of the clinic the same day.
Cost of Stem Cell Therapy for Hip Pain: Global Comparison
Stem cell therapy for Hip Pain costs vary widely by country; Japan commands a premium for its high-tech culturing and safety testing, while Turkey and Mexico offer budget-friendly alternatives.
Costs depend heavily on the cell count (number of cells injected) and the source (fat vs. bone marrow).
Detailed Cost Comparison Table
|
Feature |
Japan (Premium) |
Turkey (Value) |
Mexico (Accessible) |
USA (Limited) |
|---|---|---|---|---|
|
Avg. Price (Hip Package) |
$12,000 – $25,000 |
$3,000 – $6,000 |
$3,500 – $8,000 |
$5,000 – $12,000 |
|
Cell Type |
Cultured Adipose (Fat) |
Bone Marrow / Adipose |
Adipose / Allogeneic |
Bone Marrow Concentrate |
|
Cell Count |
100M – 200M+ |
30M – 50M |
30M – 100M |
Uncounted (Low dose) |
|
Regulation |
Strict (ASRM Law) |
Moderate |
Variable |
Strict (No culturing allowed) |
|
Recovery Stay |
2 Visits (1-2 days each) |
3-5 Days |
1-3 Days |
Same Day |
|
Ideal For |
Safety-conscious, severe cases |
Budget-conscious, combining with tourism |
US/Canada residents needing proximity |
Patients avoiding travel |
Expert Insight: "While Turkey offers incredible prices roughly 70% lower than Japan, patients must carefully verify if the clinic uses fluoroscopic guidance for hip injections. Blind injections can miss the joint space entirely, rendering the treatment useless." — Dr. S., Regenerative Medicine Specialist.
Risks, Side Effects, and Recovery
Serious complications are extremely rare with autologous stem cells; most patients experience only mild swelling or soreness at the injection site for a few days.
Recovery Timeline
-
Days 1-3: Mild soreness in the hip and harvest site. Rest is recommended.
-
Weeks 2-4: Inflammation begins to subside. You may feel "lighter" in the joint.
-
Months 3-6: Peak regeneration occurs. Significant improvements in walking distance and pain scores.
Potential Risks
-
Infection: Risk is less than 0.1% in sterile Japanese clinics.
-
Temporary Pain Flare: The injection causes a temporary inflammatory response (a sign of healing).
-
No Result: In rare cases (approx. 10-15%), patients may be "non-responders" and feel no change.
Frequently Asked Questions (People Also Ask)
Does stem cell therapy for hips really work?
Yes, clinical studies and patient outcomes show that stem cell therapy is effective for 75-85% of patients with mild to moderate hip osteoarthritis. It works by reducing inflammation and signaling the body to repair damaged tissue, delaying the need for hip replacement by years.
Is stem cell therapy better than hip replacement surgery?
They serve different purposes. Stem cell therapy is a regenerative option for those who want to save their natural joint and avoid surgery. Hip replacement is a reconstructive surgery for end-stage arthritis where the joint is destroyed. Stem cells generally have a faster recovery and fewer risks than surgery.
Why is stem cell therapy illegal or limited in the US?
It is not illegal, but the FDA classifies cultured (expanded) stem cells as a "drug," requiring years of clinical trials before approval. Therefore, US clinics can only offer "same-day" procedures with much lower cell counts. Japan allows cultured cells under its safety laws, providing more potent treatments.
How long does it take to see results from hip stem cell injections?
Most patients begin to notice a decrease in pain and stiffness between 3 to 6 weeks post-procedure. The cells continue to work and regenerate tissue for up to 12 months, with the most significant improvements usually felt around the 3-month mark.
Can stem cells cure Avascular Necrosis (AVN) of the hip?
Stem cells are highly effective for Stage 1 and 2 AVN, where they can restore blood flow and regrow bone, potentially reversing the disease. For Stage 3 (collapse), they can reduce pain but likely cannot reverse the structural deformity.
What is the difference between PRP and Stem Cell Therapy for hips?
PRP (Platelet-Rich Plasma) uses growth factors from your blood to stimulate mild healing, acting like a fertilizer. Stem cells are the "seeds" that can actually turn into new tissue. Stem cell therapy is significantly more potent and better suited for moderate-to-severe hip degeneration than PRP.
Ready to Restore Your Mobility?
Don't let hip pain dictate your life. PlacidWay partners exclusively with government-licensed clinics in Japan, Turkey, and Mexico to bring you safe, effective, and affordable regenerative solutions.
Why Book with PlacidWay?
-
Certified Safety: We verify that our Japanese partners hold valid ASRM licenses.
-
Direct Pricing: Get the same price as the clinic—no hidden agency fees.
-
Full Support: From medical visa assistance to booking your hotel and interpreter.


.png)




.png)
.png)





Share this listing